Air compressor to power artificial heart, soon to be in service
Release Date: 2017-01-22 Source: China Compressor Network View: 631
Core Tip: The human heart is an engineering marvel. In the chest cavity of an average adult, the heart will beat about 100,000 times a day. Human blood can be up to the brain, down to the fingers and toes,; behind the scenes is the role of the heart and elastic arterial tubes like a pump. The heart has four chambers, one atrium and one ventricle on each side, which are used to pump blood throughout the body. This ingenious design in nature is the envy of engineers. Since the 1950s, countless scientists have been trying to develop artificial hearts, but the results have always been poor. According to a recent report in the American magazine IEEE Spectrum,
The human heart is an engineering marvel. Inside the chest cavity of an average adult, the heart beats about 100,000 times a day. The human body's blood can travel up to the brain and down to the fingers and toes; it is the pump-like action of the heart and the elastic arterial vessels that are behind the scenes. The heart has four chambers, one atrium and one ventricle on each side, which are used to pump blood throughout the body.
This ingenious design in nature is the envy of engineers. Since the 1950s, countless scientists have been trying to develop artificial hearts, but the results have always been poor. According to a recent report in the American magazine IEEE Spectrum, four companies around the world claim that they have found the right technology. in 2017, clinical trials and animal testing may *finally* prove that a permanent artificial heart is expected to be stationed in a patient's chest cavity for a lifetime of service.
Mimicking the natural heart
Currently, about 5.7 million people in the United States alone have been diagnosed with heart failure, and many of these patients have to wait in line for heart transplants, but there are very few donors. It is reported that there are only 2,000 to 2,500 heart donors in the U.S. each year, and thousands of seriously ill patients have to wait months or even years for heart transplants to be implemented, and many die while waiting.
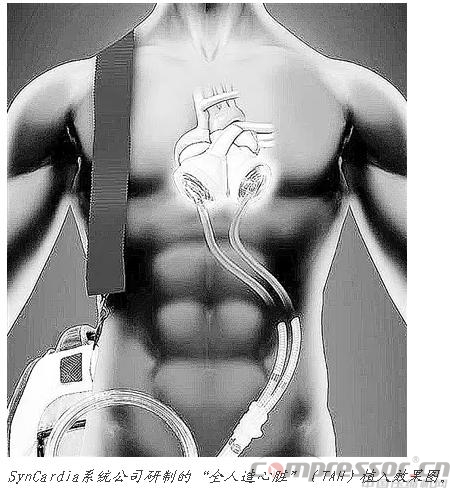
Arizona-based SynCardia Systems developed the ;Total Artificial Heart that has been vetted by U.S. regulatory agencies. Although the SynCardia TAH functions well, it can currently only be used as a ;transitional treatment prior to heart transplantation, requiring replacement of the air compressor every three months.
Now, the company is recruiting patients to start clinical trials for the device as a permanent heart replacement. Michael Gallup, the company's chief executive officer, said the trial will enroll only 28 patients because more than 1,600 implantations have already been performed to prove the artificial heart is safe.
According to Gallup, the device is simple in design and durable; there are virtually no electronics in the patient's body.SynCardia's heart mimics the heart's pumping function with two plastic ventricles; each is divided by a diaphragm into two flaps, one containing air and the other blood. Patients fitted with the SynCardia TAH carry an air compressor weighing about 6 kilograms connected to tubing that sends air through the abdomen to both chambers, pushing the diaphragm to drive blood flow through the other side. The air compressor hammers loudly at a frequency of 120 times per minute.
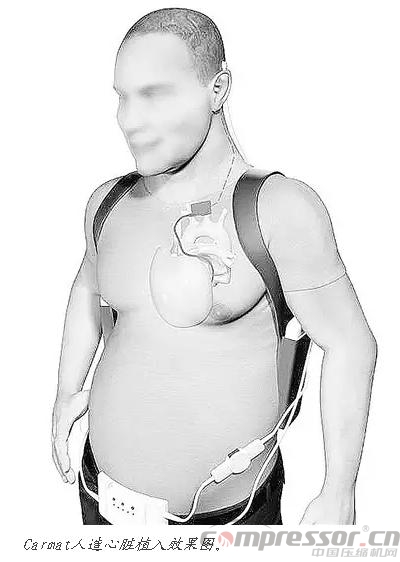
A French company called Carmat wants to do better. According to the company's chief medical officer, Pete Jansen, ;our device is silent. Like SynCardia's device, the Carmat heart has two artificial chambers with diaphragms that press outward to pump blood flow, but instead of compressed air, it uses a hydraulic fluid powered by an implanted pump. Although Carmat's artificial heart is bigger, heavier and more complex than SynCardia's device, the company's designers are extremely proud of the sensors and microprocessor that calculates changes in heart rate.
The first feasibility study of Carmat's artificial heart seems to have gone badly: two out of four patients died within three months, but industrial analyst Andrew Thompson says the patients themselves were so sick that ;rather than a failure of the device, the patient's body itself had run out of gas.
The company hopes to have the device surgically implanted in 20 patients by the end of 2017; and to have it certified as a permanent implant in Europe in 2018.
Eliminating mechanical wear and tear
The other two companies, which have not yet entered the clinical testing phase, will go the other way with a completely different technology. Some experts believe that this technology is more promising. ; Artificial mechanical devices cannot stably mimic the heart's 35 million beats per year for long periods of time without wearing out because they do not have the heart's self-repairing capabilities.
So instead of using a beating diaphragm, the companies use centrifugal pumps with fast-turning fan-shaped blades that drive a ;continuous flow of blood through the arteries.In 2015, an experiment carried out by the Cleveland Heart Company in the U.S. kept two baby calves alive and healthy for 90 days. Another company called BiVACOR is currently working with the Texas Heart Institute on an ongoing 90-day study with heifer calves.
BiVACOR's product design is simple: a rotor with two blades spins in a small titanium chamber. One small blade presses blood into the right ventricle to reach the lungs; the larger blade allows blood to flow out of the left ventricle and into the circulatory system throughout the body. The rotor is suspended in a magnetic field to further minimize wear and tear by eliminating friction, and the magnetic levitation technology controls the rotation of the blades to match the user's activity level.
This device eliminates mechanical wear and tear and malfunction at the source, and at 1/3 the size of a Carmat heart, it is energy efficient and has a high output for patients of all sizes. However, the potential drawback is that the blood produces a small amount of foam, which could lead to internal bleeding, stroke, or other complications.
The companies are still tweaking their designs in hopes of early human trials.
Heart surgeon Gianluca Torregrossa, who has implanted the SynCardia device in patients several times, is bullish on both companies.

Letting time be the judge
All four of these companies will need to conduct clinical trials in very demanding environments in order to prove that their technologies are practical and feasible. multiple clinical trials in 2017 could show us an engineering marvel created by people, not biology.
Heart surgeon William Cohen, director of the Center for Technology and Innovation at the Texas Heart Institute, said, ;Perhaps BiVACOR will prove to be a huge failure; perhaps Carmat's subsequent human trials will be a huge success, and only time will reveal the answer. As long as one of these technologies works, it is possible that patients will be spared a long and painful wait.
What is the ultimate goal of permanent TAH? Is it an alternative to heart transplantation? These are questions that only time will;judge.